Life and death have long been viewed as distinct opposites. Death marks the irreversible end of an organism’s function.
However, a new body of research suggests that cells from deceased organisms can reorganize into new multicellular structures, introducing a ‘third state’ that defies conventional boundaries between life and death.
In a fascinating development, researchers have found that cells such as skin cells from dead frog embryos can adapt to lab conditions, forming multicellular structures called xenobots.
These xenobots exhibit functions far beyond their original roles, using hair-like structures known as cilia to navigate their environments, a behavior previously restricted to moving mucus in living embryos.
Likewise, solitary human lung cells have been observed to self-assemble into multicellular entities known as anthrobots.
These anthrobots can move autonomously and even repair nearby damaged neuron cells, showcasing remarkable adaptability and self-sufficiency.
Such discoveries push the boundaries of our understanding of cell behavior. Typically, transformations in nature, such as caterpillar metamorphosis into butterflies, are predetermined.
However, xenobots and anthrobots demonstrate that cellular structures can evolve new functions even after the organism has perished, challenging the notion that death is an absolute end.
Cell survival and functionality postmortem depend on factors such as environmental conditions, metabolic activity, and preservation techniques.
For example, human white blood cells generally die within 60 to 86 hours after death, while skeletal muscle cells in mice can regenerate up to 14 days postmortem.
Cryopreservation techniques, which store bone marrow and other tissues, allow for similar functionality as those derived from living donors.
Recent studies have revealed increased activity in stress-related and immune-related genes postmortem, suggesting that cells can undergo significant changes to maintain homeostasis even after the organism’s death.
Factors such as trauma, infection, and the time elapsed since death also significantly impact cell viability.
Researchers have speculated that specialized channels and pumps in cell membranes, functioning as intricate electrical circuits, play a crucial role in postmortem cellular functionality.
These electrical signals may facilitate communication between cells, driving growth, movement, and the formation of new structures.
The implications of this ‘third state’ are vast, offering exciting prospects for medicine and biology.
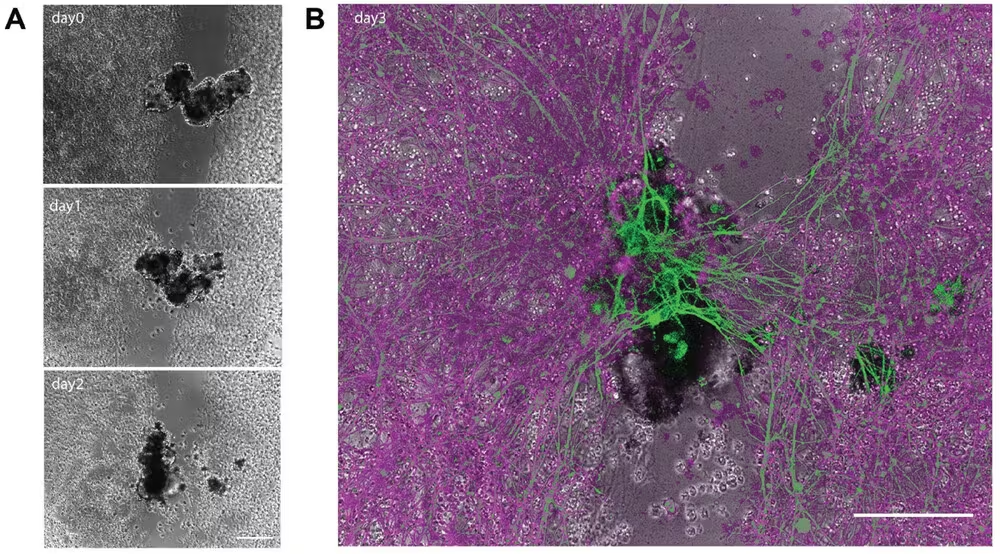
For instance, anthrobots sourced from an individual’s tissues could deliver drugs without triggering an immune response, potentially dissolving arterial plaque or removing excess mucus in patients with specific conditions such as atherosclerosis or cystic fibrosis.
These multicellular organisms have a finite lifespan of about four to six weeks, naturally degrading and preventing the risk of uncontrolled growth.
This burgeoning field not only provides new insights into cellular adaptability but also paves the way for personalized and preventive medical treatments.
By exploring how cells continue to function and transform postmortem, we may unlock new therapeutic possibilities that extend the boundaries of life and death.
