In a groundbreaking development, researchers have discovered a new method to transform carbon dioxide (CO2) into methanol using sunlight, a breakthrough that could revolutionize sustainable energy generation. This innovation comes from the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), a U.S. Department of Energy-funded consortium that includes researchers from prestigious universities like Princeton and Yale.
The research focuses on using three-dimensional silicon scaffolds on photoelectrodes to improve the yield of desired products when sunlight is used to turn carbon dioxide into liquid fuel. The technique mimics the natural process of photosynthesis in plants, effectively reducing CO2 levels in the atmosphere while generating an energy-dense fuel that can replace traditional fossil fuels like diesel and petrol. This dual benefit offers a promising solution for industries and long-haul transportation, which cannot rely solely on battery packs.
Scientists have long been aware of high-surface-area silicon as an effective material for various applications. However, it has never been used in photoelectrodes for liquid solar fuel generation until now. The CHASE research team built photoelectrodes using this high-surface-area silicon material, allowing them to examine catalysts at a molecular level and understand their role in the chemical reactions better.
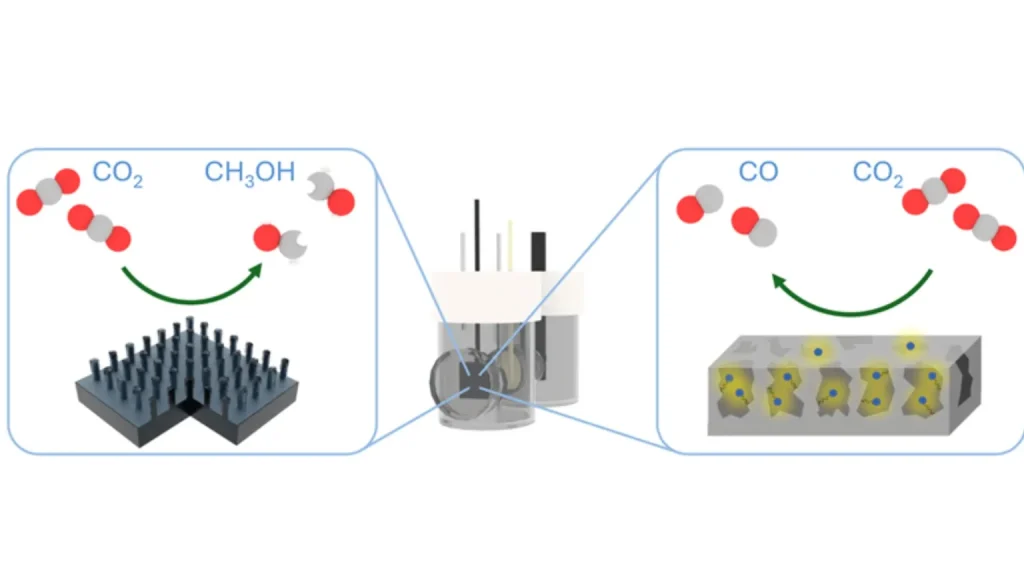
In practical terms, the team used three-dimensional silicon micropillars coated with a cobalt catalyst to produce methanol. This setup showed a higher current density, suggesting it could be an advanced photoelectrode for future liquid fuel generation. Another experiment involved using nanoporous silicon integrated with a rhenium catalyst, leading to higher durability and selectivity in converting CO2 to carbon monoxide (CO), a precursor for many other useful products.
According to the findings published in the Journal of the American Chemical Society, this novel approach can potentially increase the yield of desired chemical products, namely methanol and carbon monoxide. The use of high-surface-area silicon in hybrid photoelectrodes shows significant advantages, making it a cutting-edge method for producing liquid solar fuels.
Funding for this study came from multiple sources, including the U.S. Department of Energy’s Office of Science, the Air Force Office of Scientific Research, and the National Science Foundation. The successful integration of three-dimensional silicon scaffolds with various catalysts marks a significant step toward sustainable energy solutions, utilizing nothing but CO2, water, and sunlight.
As the technology advances, the potential for creating a closed loop of fuel usage and generation becomes increasingly feasible. This innovation can significantly contribute to global sustainability efforts, bringing us closer to a fossil-fuel-free future.
