In a remarkable development in the field of battery technology, researchers from the University of Glasgow, in collaboration with experts at Helmholtz Institute Ulm, have unveiled a breakthrough material that could herald a new era of potassium-ion batteries.
This advancement is potentially significant, considering the growing global demand for eco-friendly and cost-efficient battery technologies, driven by the increasing use of portable electronics and electric vehicles.

Potassium-ion batteries are gaining traction as a viable alternative to the well-established lithium-ion systems.
The abundant availability of potassium resources worldwide, coupled with certain advantageous properties such as rapid charging, render potassium-ion technology an attractive option.
The recent breakthrough offers hope for making these batteries not only more cost-effective but also simpler to produce.
This could open the door to expanded applications, particularly in storing energy from renewable sources.
Heading the research team, Dr. Alexey Ganin from the University of Glasgow’s School of Chemistry, remarked on the potential strategic benefits of adopting potassium-ion batteries.
“While lithium-ion batteries are celebrated for their high performance in various devices, from smartphones to electric vehicles, the scarcity of lithium highlights a need for alternative resources,” explained Ganin.
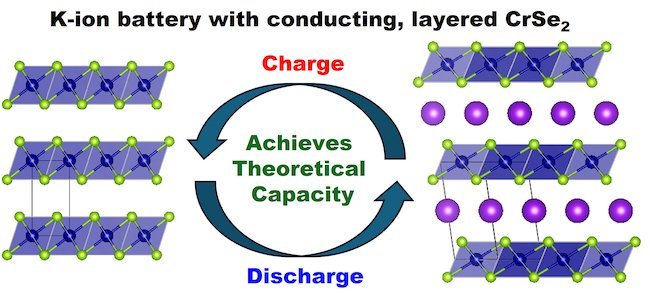
One of the major hurdles in the development of potassium-ion batteries has been the need to enhance conductivity by mixing existing cathode materials, such as Prussian White, with carbon.
However, the innovative chromium selenide material developed by the research team has shown promising results with less than 10% carbon, achieving a performance nearing its theoretical capacity limits.
The unique layered structure of the chromium selenide material facilitates the smooth movement of potassium ions during charging and discharging cycles.
This enables the battery to maintain over 85% of its capacity even at high charging rates, according to laboratory tests.
The focus now shifts to identifying an electrolyte that could further elevate the battery’s performance, as current lithium-ion batteries benefit from specialized electrolytes readily available on the market.
Dr. Ganin acknowledged the promising results while stressing the need for further research: “The right electrolyte could significantly boost performance.
We’re eager to collaborate with experts, perhaps in the field of robotics, to explore thousands of chemical combinations that could lead to the optimal solution for our batteries.”
He emphasized the potential environmental sustainability that potassium-ion cells could bring, thus supporting the quest for more sustainable energy technologies.
The groundbreaking research has gained attention across various institutions, including Ulm University, the Karlsruhe Institute of Technology, the University of Kent, and the Catalan Institute of Nanoscience and Nanotechnology.
Their collective efforts were highlighted in their published paper in the Journal of Materials Chemistry A, detailing the reversible K-Ion intercalation in CrSe2 cathodes.
This discovery not only underscores the importance of potassium-ion technology but also opens avenues for expanding the range of energy storage options available.
As the demand for renewable energy solutions intensifies, such research efforts are invaluable in fostering advancements that align with these global efforts.
